Product Details
NOx Scrubber
Nitrogen released from fuel combustion process reacts with oxygen and forms Nitric Oxide (NO). The Nitric Oxide is a colorless gas and it will be oxidized into Nitrogen dioxide (NO2) upon reacting with oxygen. This NO2 has odorous and acidic in nature, causes hazardous to human health and the environment, even in typically low concentrations.
Product CatalogueDescription
NOx Scrubber
Nox Scrubber
Nitrogen released from fuel combustion process reacts with oxygen and forms Nitric Oxide (NO). The Nitric Oxide is a colorless gas and it will be oxidized into Nitrogen dioxide (NO2) upon reacting with oxygen. This NO2 has odorous and acidic in nature, causes hazardous to human health and the environment, even in typically low concentrations.
Health Impact associated with NOx
The major health issues associated with NO2 is impact on respiratory system and lung dysfunction.
IDLH value of NO2: 20 ppm
PEL value of NO2: 2 ppm
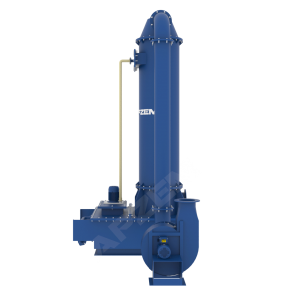
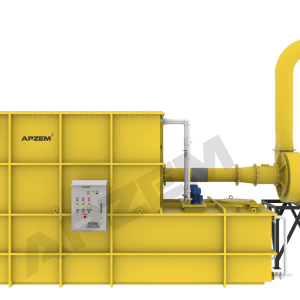
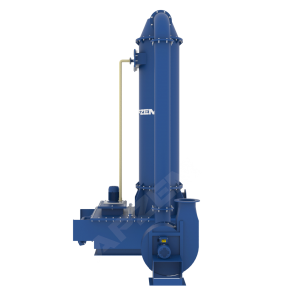
Working Principle of NOx Scrubber
It works based on the absorption with chemical reaction, involved to remove pollutants from exhaust gas by dissolving into the liquid.
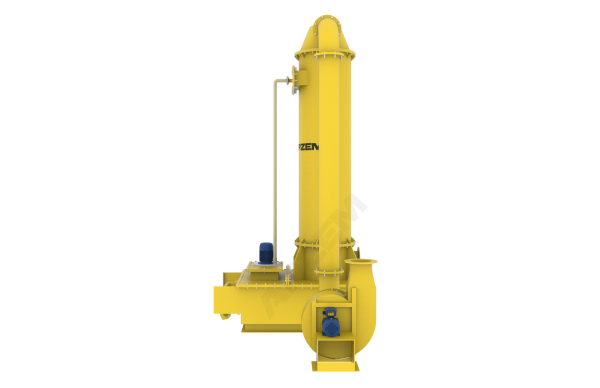
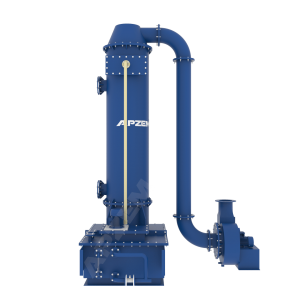
A central piece of equipment, called packed bed column, in which the polluted gas is fed from the bottom and pass upward through packing material. At the same time, solvent is sprayed from the top to wash the pollutants. Nox scrubber includes packed bed column, scrubber tank, recirulation pipe connected with solvent spray nozzle. Recirulation pump, Instrumentations, Suction blower and Control panel. All the parts involved in the scrubber unit is designed and selected based on the standard design criteria.
Packed column helps to achieve heat transfer and mass transfer. The exhaust gas contains NOx mostly has high temperature than atm. The packed column is utilized in cooling of hot gases and dissolving acid gases into the solvent. Water will be used as solvent for the physical absorption. However, Alkali solution will be used for continuous effective scrubbing. Packing material with high surface area is used to increase intimate contact between gas and liquid.
Chemical reaction
Oxides of nitrogen includes N2O, NO, N2O3, NO2, N2O4 and N2O5 generates from combustion process. The primary NOx are NO and NO2. Nitrogen dioxide (NO2) can be neutralized by absorption and chemical reaction with NaOH. However, the NO does not dissolve easily into the water. NO2 is mostly considered as NOx through the National Ambient Air Quality Standards (NAAQS) as per the EPA.
2NO2 + 2NaOH → NaNO2 + NaNO3 + H2O
- Metal finishing process
- Nitric acid manufacturing process
- Combustion process
| AIR HANDLING VOLUME |
|
| TYPE |
|
| SYSTEM PRESSURE DROP |
|
| REMOVAL EFFICIENCY |
|
| MOC |
|
| PACKING MATERIAL |
|
| HEIGHT OF TRANSFER UNIT (EFFECTIVENESS OF PACKING) |
|
| FLOODING |
|
| NOZZLES |
|
| STRUCTURE |
|
| CONTROL SYSTEM |
|
| ACCESSORIES |
|
| SAFETY ACCESSORIES
|
|
| Scrubbing liquid |
|
| Blower |
|
| Control panel |
|