Month: January 2021
Air Pollution due to Transportation
Nearly 50 % of people living in the United States—an estimated 150 million—live in areas that don’t face air quality standards. Public vehicles and heavy-duty trucks are a major source of this pollution, which includes ozone, particulate matter, and other smog-forming emissions.
Automobile such as cars, motors, taxies, trucks, scooters, buses, Lorries, aeroplane etc., are considered as primary sources for transportation. CO, NO, NO2 gases released by the internal combustion engines of cars, buses, trucks, aero planes etc. cause air pollution. The released of pollutant gas by the internal combustion engine where petrol is used as a fuel.
Let us consider petrol is used as fuel in car engine. Petrol contains hydrocarbons as main constituent. These hydrocarbons have the general formula C8H18 and hence called octanes. petrol burns quickly in a car engine .Due to the short time available for burning, incomplete combustion of petrol takes places and so CO, CO2, H2O vapor alcohol, and acid, unburnt carbon particles are released. CO and carbon particles emitted into the air and make it polluted.
The automobile exhaust are responsible for more than 75 per cent of total air pollution. The automobiles such as cars, scooters, taxis etc. release smoke which contain huge amount of poisonous gases .These gases are released as a result of incomplete combustion of petrol and diesel, causing air pollution. Is named as anti – Knocking petrol. Anti-knocks reduce the rate of combustion of the fuel. The Chloro Fluorocarbons released from supersonic aeroplanes are responsible for ozone depletion.
It was assumed that throughout the world the total number of vehicles like cars, buses, motor vehicles running on the road per a day may be 500 million (5*108). Out of these vehicles 2.5 million are running on the roads of our country India. Due to this reason per one day 800 to 1000 pollutant gases are entering into the atmosphere causing air pollution.
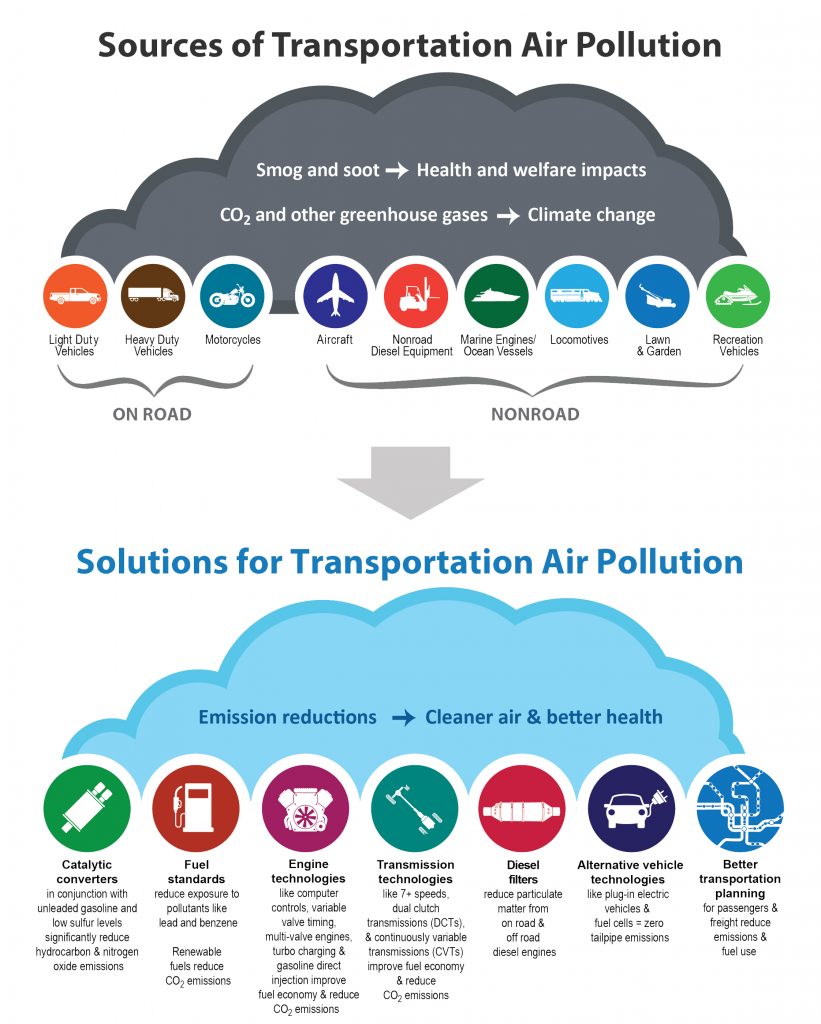
Transportation and Climate Change
Burning of fossil fuel such as Coal, Oil, Gasoline release carbon monoxide, a greenhouse gas, carbon monoxide, hydrocarbons, nitrogen oxide, and particulate matter into the atmosphere. The release of carbon monoxide and other greenhouse gases like methane Nitrous oxide and hydro fluorocarbons is causing the atmospheric earth to warm , that the result are changes to the climate .
The Emitted pollutants from Fuels, which are harmful as smoking 10 cigarettes a day. When the vehicle pollution is high in the atmosphere, it creates a hole in the ozone layer contributing to smog and causing various health issues.
Greenhouse gas (GHG) emissions from transportation account for about 28 percent of total U.S. greenhouse gas emissions, making it the largest contributor of U.S. GHG emissions. Between 1990 and 2018, GHG emissions in the transportation sector increased more in absolute terms than any other sector.

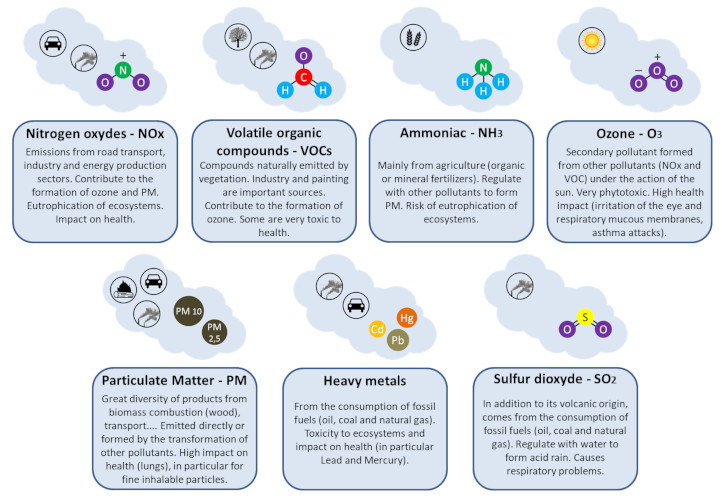
Particulate Pollutants
Pollutant is a substance present in nature, in greater than natural abundance due to human activity. Which ultimately has a detrimental effect on the environment and therefrom on living organisms and mankind. For example lead, mercury, sulphur dioxide, carbon monoxide. Etc.
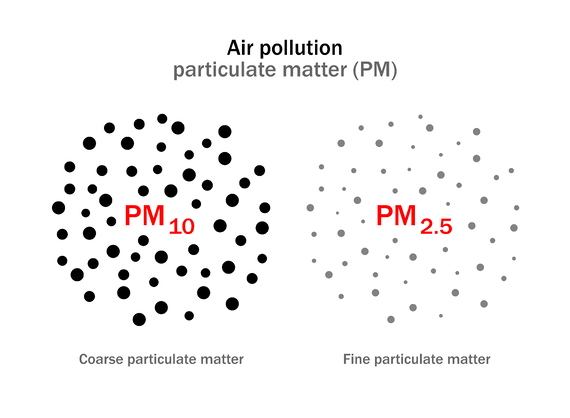
Air pollution due to particulate matter
Thus, small solid particles and liquid droplets are collectively called as particulates. Particulates are present in the atmosphere in fairly large numbers and pose a serious air pollution. They are classified, in accordance with particle size and nature, into different types such as fumes, dust, ash, carbon, smoke, lead, asbestos, mist, spray, oil, grease etc.
Formation of Particulate Pollutants
Natural Formation
Natural processes like volcanic eruptions, wind and dust storms, salt spray etc., release particulates into the atmosphere. Nearly 2000 million tonnes of particulate matter is entering into the atmosphere from natural resources per every one year.
Man-Made Particulate Pollutants
Manmade activities also inject 450 million tonnes of particulates into the atmosphere which causes air pollution. Dust and asbestos type of particulate pollutants enters into the atmosphere during the construction. Similarly fly ash from power plants, smelters, mining processes and smoke due to the incomplete (or) partial combustion of fuel etc., are also man made sources for particulate pollutants.
Particulate pollutants may be classified according to their nature and sizes, as follows:
Dust :
Dust is formed by solid particles and their size range from I micron to 100 microns. Dust particles with a size of 0. 1 micron are also present in the atmosphere. Dust panicles are:
- Entrained by gases directly from the materials being handled or processed, E.g. coal
- The off-spring obtained directly from the parent material when it undergoes a mechanical operation. E.g . Saw-dust from works;
- Entrained materials used in mechanical operations, E.g. sand From sand blasting .
Fume :
The size of these particles are less than I micron. Fumes are generally formed from particles of the metals and metallic oxides. Fumes are formed by the condensation of vapors by sublimation, distillation, calcination and by other chemical processes and chemical reactions.
Spray :
Liquid particles obtained from the parent liquid by the mechanical disintegration processes such as atomization is known as Spray.
Smoke :
Smoke is a collection of tiny solid, liquid and gas particles. Although smoke can contain hundreds of different chemicals and fumes, visible smoke is mostly carbon (soot), tar, oils and ash.
Secondary Pollutants
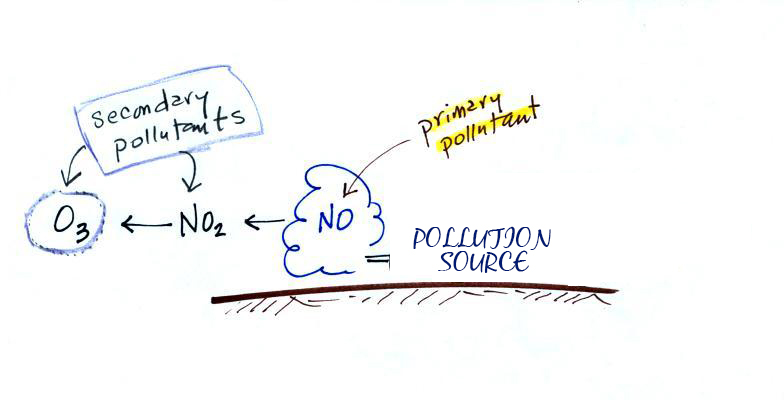
All the gaseous pollutants are primary pollutants. These pollutants are emitted directly and such are not found in the atmosphere. Instead, they form as a result of the pollutants emitted from these sources reacting with molecules in the atmosphere. Pollutants that are emitted into the environment from a source called Primary pollutants. There are also secondary pollutants which are formed in the atmosphere.
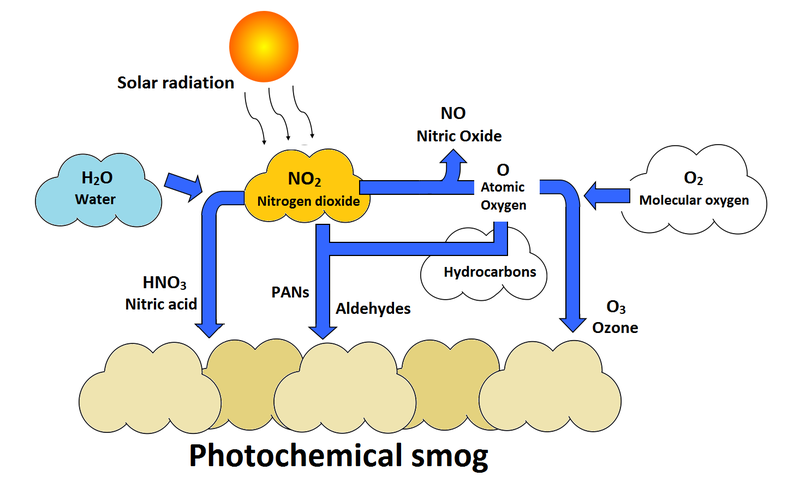
Secondary pollutants are those which are formed in the air due to interaction of primary air pollutants among themselves or by reaction with normal atmospheric constituents like sunlight, water vapor etc. With or without photo activation. For example ozone, formaldehyde, peroxy – acetyl nitrate and acidic mist like H2SO4 (SO2 + moisture), smog, photochemical smog.
Evidences and experiments indicate that exhaust gases of automobiles have particular importance in the formation of secondary pollutants For example
Nitrogen oxide produced in the combustion of petroleum and other fuels emitted to the atmosphere, yield ozone in the presence of sunlight. It is to be noted that ozone is not emitted as such to the atmosphere but formed only from primary pollutants by the interaction in the atmosphere and thus constitute of secondary pollutants. The ozone produced reacts with hydrocarbons to form a series of compounds such as aldehydes, ketones, organic acids, acyl nitrates and epoxy compounds. This types of photochemical reactions usually occur in smog. The term ‘ smog’ denotes a mixture of smoke and fog .it is reported that during a smog , the concentration of ozone and oxidant material are found to be high . According to the Haagen – smit theory , ultraviolent rays in the sunlight break the nitrogen dioxide molecule to form nitric oxide and atomic oxygen . These products react with molecular oxygen to form ozone. The reaction may be represented as follows
- NO2 – NO + O
- O2 + O – O3
- NO + O2 – NO3
- NO3 + O2 – NO2 + O3
- 2NO2 + O3 – N2O5 + O2
- NO + O3 – NO2 + O2
The liberated molecular oxygen reacts with molecules, of aldehydes and Oxy- genated organic compounds. which are activated by the absorption of light .
RCOOOH+O2 – RCOOH + O2
Thus once again ozone is liberated as a by product. On absorption of light energy, some of the aldehydes and ketones become free radicals. They react with molecular oxygen as follows
HCO + O2 – HCO3
The free radical HCO3, reacts further with molecular oxygen, nitric oxide and hydrocarbons as Follows
HCO3 + O2 – HCO2 + O3
HCO3 + NO – HCO2 + NO2
The cycle reaction is set in motion and numerous products are formed. It is to be noted that ozone and nitrogen dioxide are evolved continuously throughout the cycle of reaction. Free radicals lead to several other types of free radicals reaction and polymerization and oxidation chain reaction.
Though these reaction are studied well, the chemistry of photochemical smog is still not clearly understood. Many attempts have been made to reduce the level of ozone in the atmosphere. Actually, if the amount of hydrocarbons released in the atmosphere is reduced, then the ozone formed will be utilized in the oxidation of nitric oxide to nitrogen dioxide. But unfortunately the level of ozone have never decreased and the puzzle has not yet been solved .
Primary Pollutants

Outdoor air is filled with millions of particulate pollutants. Apzem provides the industry best experts who perform Air Quality Monitoring Services for Stack, Indoor & Ambient Environment .
Air is not clean in nature, because of natural and man- made pollution. Gases such as CO, SO2 and H2S are continuously released into the atmosphere through natural resources Ex: Volcanic activity, Vegetarian decay and forest fires. Air are distributed from the tiny particles of solids and liquids by wind, Volcanic explosion and other natural explosion.
The main sources of air pollutants are primary pollutants, Secondary pollutants, and Particulate Matter.
CARBON MONOOXIDE
Carbon monoxide is released into the atmosphere mainly from the automobile fume, smoke, and vapor. Next to carbon dioxide. Carbon monoxide is considered to be the most abundant pollutant and it may show wide diurnal variations in the urban atmosphere. Concentrations of carbon monoxide vary, depending upon the density of motor traffic. However. Carbon monoxide is usually present in amounts far below the threshold concentration in areas where traffic is less.
SULPHUR DI-OXIDE
Sulphurdioxide is one of the principal contaminants of air. Sulphur dioxide has its origin in the combustion of sulphur being fossil fuels. It is also present in appreciable quantities in air where coal is used as a fuel. Electric power plants. Sulphur dioxide is also released from smelters where sulphur as an ore is roasted e.g., in copper. Lead and Zinc smelting industries. Oil refineries. Sulphuric acid manufacturing industries. Fertilizer industries and paper and pulp industries give out significant amounts of sulphur di oxide Smelting operations are reported to cause heavy damage to agricultural and forest areas. Since sulphur di oxide is readily absorbed by water surfaces, soil and vegetation, deterioration and corrosion affect materials such as metals, paper, paints leather, textiles, cement, and other building material.
HYDROGEN & ORGANIC SULPHIDE
Sulphide causes odour nuisance when present even in very low concentration .However, there are not released in appreciable quantities by industrial operation since the effluents are treated before they are exposed to the atmosphere. Natural Gas refining manufactures of coke, manufacture of paper, distillation of tar and petroleum , manufacture of viscose rayon and some other chemical processes produce hydrogen sulphide.
HYDROGEN FLUORIDE
Hydrogen Fluoride and other volatile fluorides are considered to be serious pollutants even where present in concentration of 0.001 ppm by volume. Fluorides are liberated mainly from aluminum smelting industries Moreover, fluorides have the capacity to etch glass and can thee, cause considerable material destruction
HYDROGEN CHLORIDE
The concentrations of hydrogen chloride in the atmosphere are found to be less. However, higher concentrations can cause damage to vegetation and also to properties.
NITROGEN OXIDE
The primary, source of oxides of nitrogen is automobile exhaust. These are also produced as by product of some chemical industries such as manufacture of nitric acid, manufacture of sulphuric acids manufacture of nylon intermediates. It is reported that the products of combustion of natural, contain up to 50 ppm of nitrogen. The oxides of nitrogen are the second most abundant atmospheric pollutants. These are extremely dangerous to human health. The effects are sometimes more severe than carbon monoxide.
ALDEHYDE & ORGANIC ACID
These are present in lower concentrations in the atmosphere. The incomplete combustion of petroleum fuels and incomplete oxidation of lubricating oils from aldehydes and organic acids combustion of natural gas may also contribute to the formation of these materials.